Centralized sterile supply storage in procedural areas where large volumes of sterile supplies are kept require a program for management of proper temperature and relative humidity rh levels in accordance with the facilities guidelines institute fgi guidelines for design and construction of hospitals and outpatient facilities.
Sterile supply storage temperature and humidity.
60 65 degrees f sterilizer access room 24 29 degrees c.
In addition sterile supplies should never be stored near sources of moisture e g sinks exposed water pipes nor should they be stored against an outside wall.
819 the floors and walls should be constructed of materials capable of withstanding chemical agents used for.
75 85 degrees f or as recommended by the equipment sterilizer manufacturer sterile storage and.
Some examples of sterile storage areas include.
Soil can also compromise sterility.
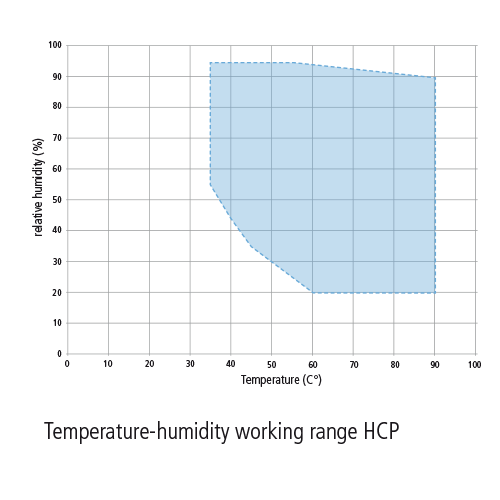
The ideal relative humidity is 50 not below 35 8 this humidity needs to be maintained to prevent absorbent material such as nonwoven and woven wrappers and peel pouches and biological bis and chemical indicators cis from drying out.
The upper limit of 70 is only for dedicated sterile storage areas all other areas e g.
The 1996 1997 2001 and 2006 editions of the guidelines list the humidity levels for sterile storage as maximum 70 percent rh.
Please see ansi aami st79 2017 comprehensive guide to steam sterilization and sterility assurance in health care facilities for more information sections 3 2 1 1 3 3 5 5 and 3 3 6 1 1.
Previously aami had recommended the following temperature and humidity levels.
Items frequently found in sterile processing requiring a specific temperature and or humidity range include wrapping materials biological indicators chemical indicators and bowie dick tests.
The humidity in sterile storage should never exceed 70.
Levels must be kept in line with the facilities guidelines institute s guidelines for design and construction of health care facilities.
Excessive humidity levels in the sterile storage area or handling packages too soon after sterilization can also affect sterility.
Humidity should be between 30 to 60.
By its very nature the risk assessment will inform all team members of the appropriate response to the parameter variance and ensure patient safety.
The sterile storage area should be a limited access area with a controlled temperature may be as high as 75 f and relative humidity 30 60 in all works areas except sterile storage where the relative humidity should not exceed 70.
Looking at the 2010 and 2014 fgi guidelines sterile storage is a subheading of central medical and surgical supply and requires humidity levels to not exceed 60 percent rh.
Reprocessing depts have the upper limit for humidity as 60.
Where can i find information on humidity ventilation or temperature.